Guided Bone Regeneration (GBR) uses the body's regenerative capability to reconstruct lost bone. Compared to other regenerative techniques, GBR has proved to be highly dependable.1 Predictable results can be obtained both functionally and esthetically, even in complex cases.
With the proven combined application of Geistlich Bio-Oss® and Geistlich Bio-Gide® as a part of a GBR procedure, clinicians can be confident that they are using complementary products that have extensive scientific documentation and proven safety and efficacy. Due to increased long-term implant survival rate2, excellent esthetic results3 and ease of use Geistlich Bio-Oss® and Geistlich Bio-Gide® are considered the standard therapy for a wide range of indications3.
Geistlich Bio-Oss®: Micropores (5000x)12
Geistlich Bio-Gide® is a unique bi-layer collagen membrane comprising a smooth and a rough, open-pored, layered surface.
Ideal environment for new bone formation4 Geistlich Bio-Oss® consists of a unique inter-connecting pore system. The micro-pores ensure an efficient fluid intake. The macro-pores permit the migration of cells.
The smooth layer facing the soft-tissue, favors the growth of fibroblasts 1 as the barrier function prevents the ingrowth of soft-tissue into the newly forming bone beneath.
The natural collagen structure of Geistlich Bio-Gide® permits prompt and homogeneous vascularization and so brings about optimum tissue integration and wound stabilization.
The rough layer facing the bone, functions as a 3-dimensional scaffold for osteoblasts 2 .
The pore system and surface morphology of Geistlich Bio-Oss® encourages the growth of osteoblasts. The porous structure serves as a scaffold for in-growing blood vessels and bone growth.
Geistlich Bio-Gide® provides an optimum barrier that integrates with surrounding tissues to protect the initial coagulum and then optimally degrades. This allows the natural complex structure of the soft tissue, with all of the intrinsic components, such as the periosteum, to form.
Due to its unique biofunctionality, Geistlich Bio-Oss® is integrated into the natural bone remodelling process. Geistlich Bio-Oss® and the newly formed bone make a stable scaffold which results in long-term clinical success.
The slow resorption of Geistlich Bio-Oss® and the ideally matched protective function of Geistlich Bio-Gide® promote the long-term volume stability14 of the augmentation material and ensure high implant survival rates.2
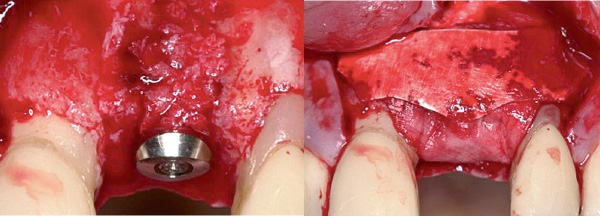

"GBR with biomaterials from Geistlich succeeds aesthetically and functionally, is extremely reliable and features minimal risk of aesthetic complications."
— Prof. Daniel Buser, Bern, Switzerland


References
1Aghaloo TL et al., Int J Oral Maxillofac Implants 2007; 22(suppl):49–70
2Jung R et al., Clin Oral Implants Res. 2013 Oct;24(10):1065–73
3Buser D et al., J Dent Res. 2013 Dec;92(12 Suppl):176S–82S
4Berglundh T et al., Clin Oral Implants Res 1997; 8(2): 117–124
5Becker J et al., Clin. Oral Implants Res. 2009; 20(7): 742–93
6Schwarz F et al., Clin Oral Implants Res. 2014 Sep;25(9):1010–5
7Rothamel D et al., Clin. Oral Implants Res. 2005;16:369–37
8 Weibrich G et al., Mund Kiefer Gesichtschirurg 4, 2000; 148–152
9 Degidi M et al., Oral Dis. 2006 Sep; 12(5): 469–475
10Orsini G et al., J Biomed Mater Res, B: Appl Biomater 74B, 2005; 448–57
11Piattelli M et al., Int J Oral Maxillofac Implants 1999; 14: 835-40
12Sartori S et al., Clin Implants Res 2003; 14: 369–72
13Traini T et al., J Periodontol. 2007 May; 78(5): 955–961
14Mordenfeld A et al., Clin Oral Implants Res. 2010 Sep;21(9):961–70