In combination, Geistlich Bio-Oss® and Geistlich Bio-Gide® are the ideal biomaterials for Minor Bone Augmentation procedures.
Geistlich Bio-Oss® provides a stable scaffold for bone formation leading to long-term volume preservation, while Geistlich Bio-Gide® ensures undisturbed bone regeneration and prevents soft-tissue ingrowth.
Learn More at Geistlich Biomaterials:

"GBR with biomaterials from Geistlich succeeds esthetically and functionally, is extremely reliable, and features minimal risk of esthetic complications."
— Prof. Daniel Buser, Berne, Switzerland

Objectives
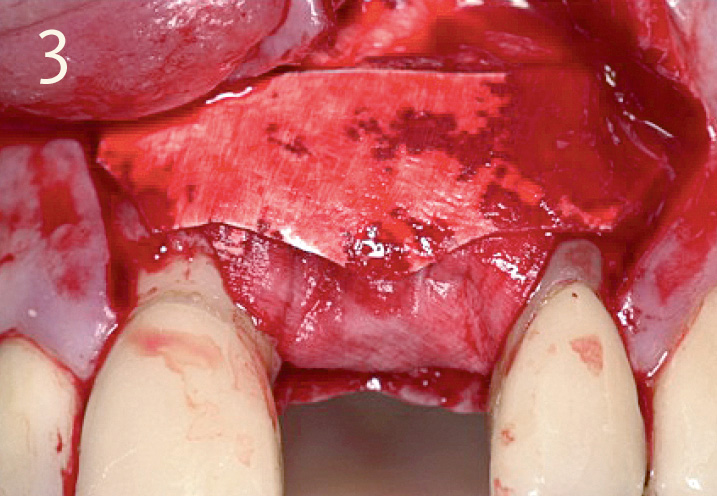
Obtain soft-tissue healing over 4 – 8 weeks, in order to achieve an intact soft-tissue covering
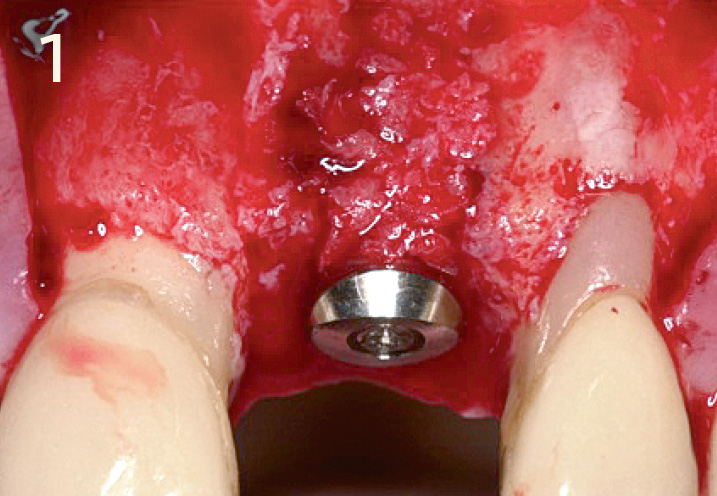
Obtain the correct 3-dimensional position of the implant during and after implant placement
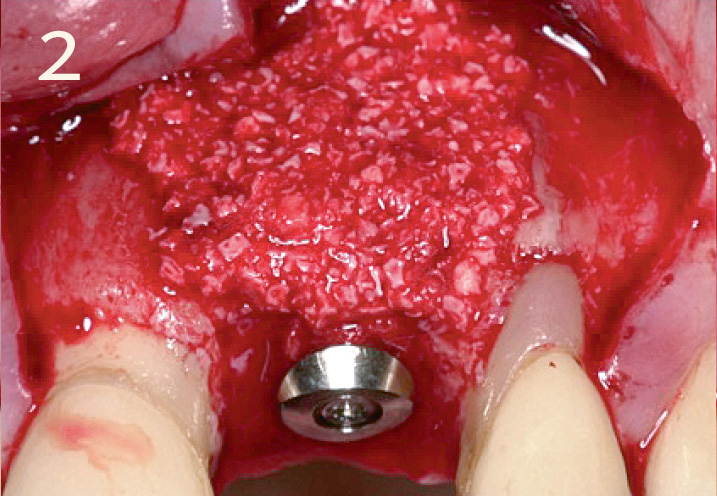
Local contour augmentation in the facial region with autogenous bone chips, Geistlich Bio-Oss® granules and Geistlich Bio-Gide® Primary wound closure with 6 – 8 week healing phase
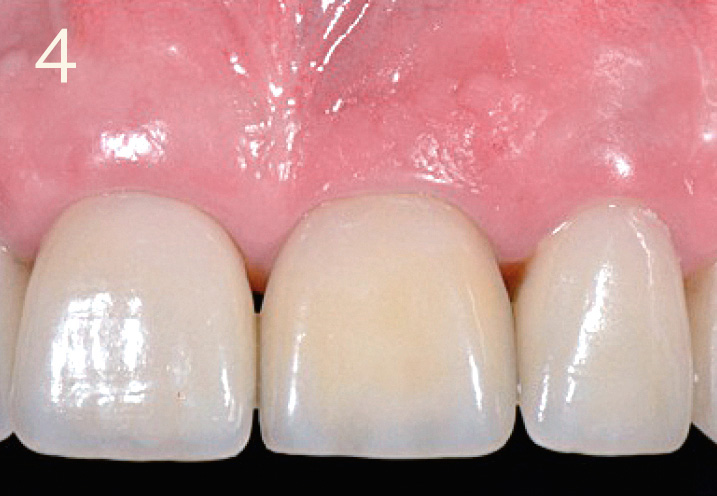
Conclusions
Esthetic restoration with screw-retained implant crown
DOCUMENTED: More than 1,000 publications
RELIABLE: 29 years of clinical experience
EXPERIENCED: 163 years of Geistlich collagen competence
CAUTION: Federal law restricts these devices to sale by or on the order of a dentist or physician.
Indications:
Geistlich Bio-Oss® is indicated for the following uses: augmentation or reconstructive treatment of the alveolar ridge; filling of periodontal defects; filling of defects after root resection, apicoectomy, and cystectomy; filling of extraction sockets to enhance preservation of the alveolar ridge; elevation of the maxillary sinus floor; filling of periodontal defec ts in conjunction with products intended for Guided Tissue Regeneration (GTR) and Guided Bone Regeneration (GBR); and filling of peri-implant defect in conjunction with products intended for GBR.
Warnings:
Possible complications which may occur with any surgery include swelling at the surgical site, flap sloughing, bleeding, local inflammation, bone loss, infection or pain.
Indications:
Geistlich Bio-Gide® is indicated for the following uses: augmentation around implants placed in immediate and delayed extraction sockets; localized ridge augmentation for later implantation; alveolar ridge reconstruction for prosthetic treatment; filling of bone defects after root resection, cystectomy, removal of retained teeth; GBR in dehiscence defects; and GTR procedures in periodontal defects.
Warnings:
As it is a collagen product, allergic reactions may not be totally excluded. Possible complications which may occur with any surgery include swelling at the surgical site, flap sloughing, bleeding, dehiscence, hematoma, increased sensitivity and pain, bone loss, redness, and local inflammation.
For more information on contraindications, precautions, and instructions for use, please visit: www.geistlich-na.com/ifu
1 iData Research Inc., US Dental Bone Graft Substitutes and other Biomaterials Market, 2012
2 iData Research Inc., European Dental Bone Graft Substitutes and other Biomaterials Market, 2012
Geistlich Bio-Oss®
Geistlich Bio-Gide®
Customer Care Toll-free 855-799-5500
Geistlich Pharma North America, Inc.
202 Carnegie Center
Princeton, NJ 08540
G-332-15E